01 October, 2022
This year Fortress Diagnostics are celebrating Lupus Awareness Month, placing a focus on the most common form of lupus, systemic lupus erythematosus (SLE). SLE is a complex and chronic autoimmune disease characterised by the appearance of autoantibodies against cytoplasmic and nuclear antigens and multi-organ inflammation 1. SLE affects 20-50 in every 100,000 individuals and is more commonly observed in women, with more than 90% of cases occurring in women, and begins at childbearing age 2, 3.
Pathogenesis of SLE
The etiopathogenesis of SLE is controversial, limiting therapeutic interventions. What is understood is that SLE is a DNA degradation and elimination disorder caused by uncleared histones and nuclear material which leaks into the extracellular space, forming cell-free DNA. This in turn triggers an immune response, destroying tissues and organs. Under homeostasis conditions, apoptosis enables DNA and other nuclear materials to be efficiently cleared through degradation and additional complex mechanisms so that this material does not trigger an immune response, which in SLE, produces nuclear autoantibodies 4.
Early symptoms of SLE commonly involve the joints and skin, disease morbidity and mortality associated with cardiovascular events (CVE) due to chronic inflammation, damage to major organs, in particular the kidneys, nervous system and hematopoietic organs, and infections resulting from immunosuppressant treatment. SLE is further characterised by a myriad of immune system aberrations, including immune complex deposition, immune system infiltration within damaged organs and pathogenic autoantibody production. The core autoantibodies present in the serum of patients with SLE are directed against nuclear components, including double-stranded DNA (dsDNA), histones, ribonucleoproteins and more. Systemic tissue damage is commonly the consequence of inflammation caused by the direct autoantibody-mediated tissue damage and the deposition of complement-fixing immune complexes 3.
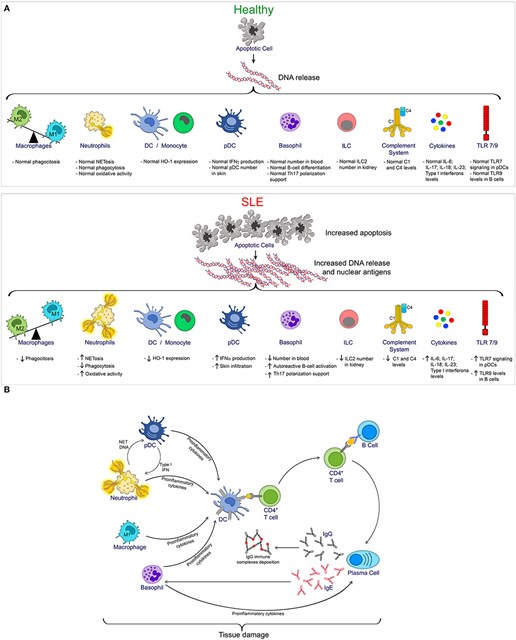
Figure 1 (A) provides an overview of the innate immune cells and pathways compromised during SLE progression. During homeostasis, controlled apoptotic cell death is rapidly cleared, reducing the exposition of nuclear antigens, thus reducing the risk of autoimmunity. Conversely, increased apoptosis as seen in SLE, combined with defective clearance, increases the exposition of DNA and nuclear antigens, which in turn, promotes the activation of multiple innate immune cells and pathways, which contributes to the pathogenesis of SLE. Figure 1 (B) provides an overview of the innate and adaptive immune cell interactions during SLE, resulting in tissue damage 3.
Figure 1: The Innate and Adaptive Immune Functions in SLE 3

Light Chains in SLE
Serum free light chains (FLC) are useful biomarkers for disease severity and flare in SLE patients 5, 6, 7. During immunoglobulin synthesis light chains, kappa (κ) and lambda (λ) light chains, and heavy chains are produced. During homeostasis, a few extra light chains are produced, which don’t bind to heavy chains and are released into the bloodstream. These unbound light chains are identified as FLC. In SLE, abnormal levels of serum FLC have been reported 8, 9. Several publications support FLC, κ:λ ratio as biomarkers of disease severity in SLE patients.
Serum free light chains as biomarkers for systemic lupus erythematosus disease severity (2011) 7
A USA study prospectively examined 75 SLE patients and 41 age- and sex-matched rheumatoid arthritis (RA) controls to determine if FLC are a putative biomarker of SLE activity. The study found that FLC levels strongly correlate with disease severity in SLE, but not in RA. The study concluded that serum FLC may be used as a biomarker or disease activity in SLE.
Determination of serum free light chains as a marker of systemic lupus flare (2019) 5
A prospective cohort study evaluated 46 patients with SLE flare to explore FLC in serum and as a biomarker of flare in patients with SLE and to analyse the difference in their discriminatory capacity with complement C3 and C4. The study concluded that λ light chains offered a good discriminatory capacity for SLE flare and could be a useful biomarker of SLE exacerbation.
Different roles of urinary light chains and serum light chains as potential biomarkers for monitoring disease activity in systemic lupus erythematosus (2022) 10
A Chinese study prospectively examined the differentially expressed proteins in urine samples between active SLE and stable SLE and to further explore the expression of light chains. The study concluded that the measurement of light chains would aid in monitoring SLE disease activity. Moreover, the study highlighted that serum light chains offered better discriminatory capacity compared to urinary light chains.
Fortress Diagnostics Light Chains Product Portfolio
At Fortress, we offer immunoturbidimetric light chain assays to aid in the diagnosis and monitoring of SLE disease activity.
References
- Pathak S, Mohan C. Cellular and molecular pathogenesis of systemic lupus erythematosus: lessons from animal models. Arthritis Research Therapy 2011; 13(241): . https://arthritis-research.biomedcentral.com/articles/10.1186/ar3465 (accessed 15 September 2022).
- Bartels CM. Systemic Lupus Erythematosus (SLE). https://emedicine.medscape.com/article/332244-overview (accessed 15 September 2022).
- Herrada AA, Escobedo N, Iruretagoyena M, Valenzuela RA, Burgos PI, et al. Innate Immune Cells' Contribution to Systemic Lupus Erythematosus. Frontiers in Immunology 2019; 10(772): . https://www.frontiersin.org/articles/10.3389/fimmu.2019.00772/full (accessed 15 September 2022).
- Arneth B. Systemic Lupus Erythematosus and DNA Degradation and Elimination Defects. Frontiers in Immunology 2019; 10(1697): . https://www.frontiersin.org/articles/10.3389/fimmu.2019.01697/full (accessed 16 September 2022).
- Rodríguez-Cambrón AB, Jiménez-Jiménez J, Blázquez-Cañamero MA, Pazos FR, Macía-Villa C, et al. Determination of serum free light chains as a marker of systemic lupus flare. Clinical Rheumatology 2020; 39(): . https://link.springer.com/article/10.1007/s10067-019-04827-4 (accessed 16 September 2022).
- Rodriguez Cambron AB, Jimenez Jimenez J, Blazquez Cañamero MA, García De Yébenes MJ, Rey Pazos F, et al. Rheumatic Diseases. FRI0344 Serum free light chains as a flare biomarker in systemic lupus erythematosus 2018; (): . https://ard.bmj.com/content/77/Suppl_2/708.1 (accessed 16 September 2022).
- Aggarwal R, Sequeira W, Kokebie R, Mikolaitis RA, Fogg L, et al. https://ard.bmj.com/content/77/Suppl_2/708.1. Arthritis Care & Research 2011; 63(6): . https://onlinelibrary.wiley.com/doi/epdf/10.1002/acr.20446 (accessed 16 September 2022).
- Martín-Nares E, Saavedra-González V, Fagundo-Sierra R, Santinelli-Núñez BE, Romero-Maceda T, et al. Serum immunoglobulin free light chains and their association with clinical phenotypes, serology and activity in patients with IgG4-related disease. Scientific Reports 2021; 11(1832): . https://www.nature.com/articles/s41598-021-81321-5 (accessed 19 September 2022).
- Medline Plus. Free Light Chains. https://medlineplus.gov/lab-tests/free-light-chains/ (accessed 19 September 2022).
- Jiang J, Zhao J, Liu D, Zhang M. Different roles of urinary light chains and serum light chains as potential biomarkers for monitoring disease activity in systemic lupus erythematosus. Peer J 2022; 10(13385): . https://peerj.com/articles/13385.pdf (accessed 19 September 2022).